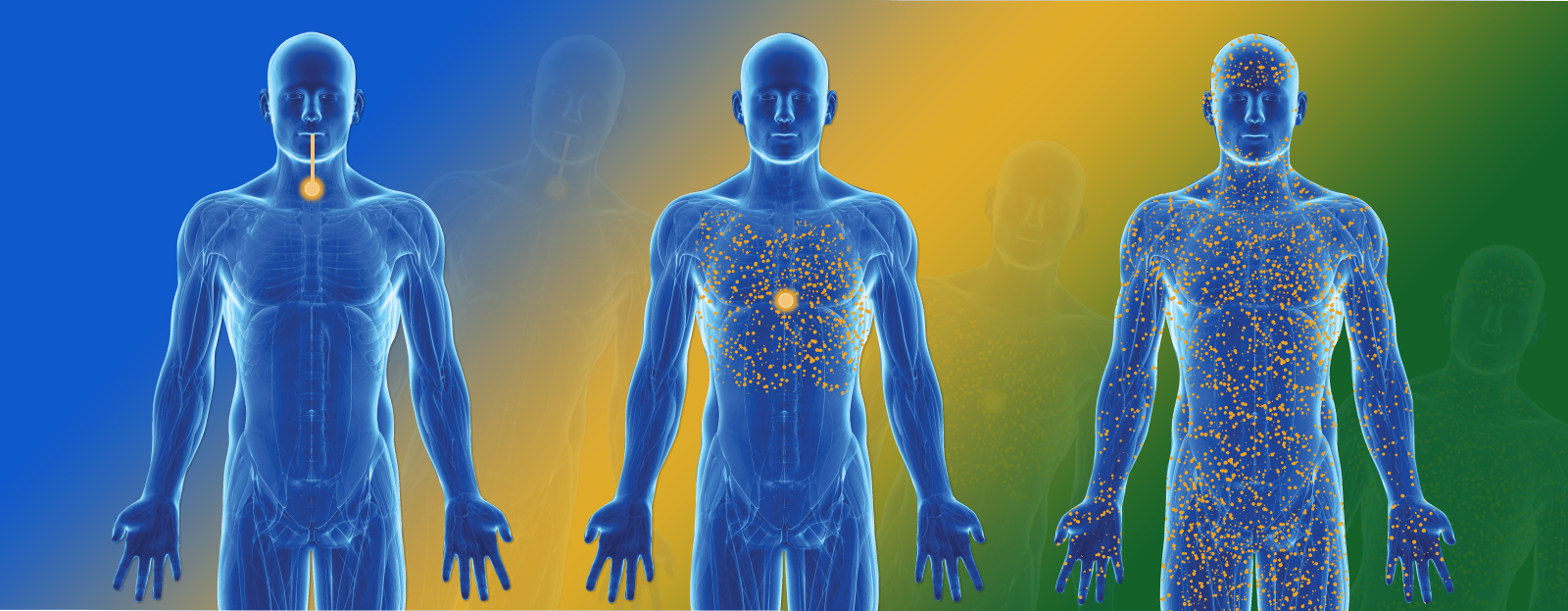
Bioavailability
Bioavailability is the measurement of the proportion of the total administered dose of a therapeutically active drug that reaches the systemic circulation and is therefore available at the site of action.
Bioequivalence
Bioavailability and bioequivalence studies play a major role in the development of both new drug products and their generic equivalents.
Bioequivalence is a term used in pharmacokinetics when there are two or more medicinal products (proprietary preparations of a drug), containing the same active substance that needs to be compared in vivo for biological equivalence.
These comparative studies are used to assess if the new version (generic) produces the same concentration in the systemic circulation when given to human participants. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same. BE studies are used as surrogates for clinical effectiveness data for generic drugs where no clinical difference is anticipated between the two products.
In determining bioequivalence, for example, between two products such as a commercially available brand product and a potential to-be-marketed generic product, pharmacokinetic studies are conducted whereby each of the preparations is administered in a cross-over study to volunteer subjects, generally healthy individuals but occasionally in patients. Serum/plasma samples are obtained at regular intervals and assayed for parent drug (or occasionally metabolite) concentration. Occasionally, blood concentration levels are neither feasible nor possible to compare the two products (e.g. inhaled corticosteroids), then pharmacodynamic endpoints rather than pharmacokinetic endpoints are used for comparison.
Bioavailability and bioequivalence studies play a major role in the development of both new drug products and their generic equivalents.