Pharmacodynamic Assessment of VRT001-C (Oral Ceftriaxone) vs. Intravenous Ceftriaxone Against Escherichia coli in the Neutropenic Murine Thigh Infection Model
The poster presentation, titled "Pharmacodynamic Assessment of VRT001-C (Oral Ceftriaxone) vs. Intravenous Ceftriaxone Against Escherichia coli in the Neutropenic Murine Thigh Infection Model" was presented on April 13, 2019, in ECCMID 2019 at Amsterdam, Netherland. VRT001-C (Oral Ceftriaxone) is based on VMRC proprietary “Stealth Targetting Nanoparticle (STN)” platform technology. STN technology employs a polymeric backbone and a targeting moiety for the oral delivery of poorly bioavailable drugs that belong to Biopharmaceutical Classification System (BCS) Class III and IV.
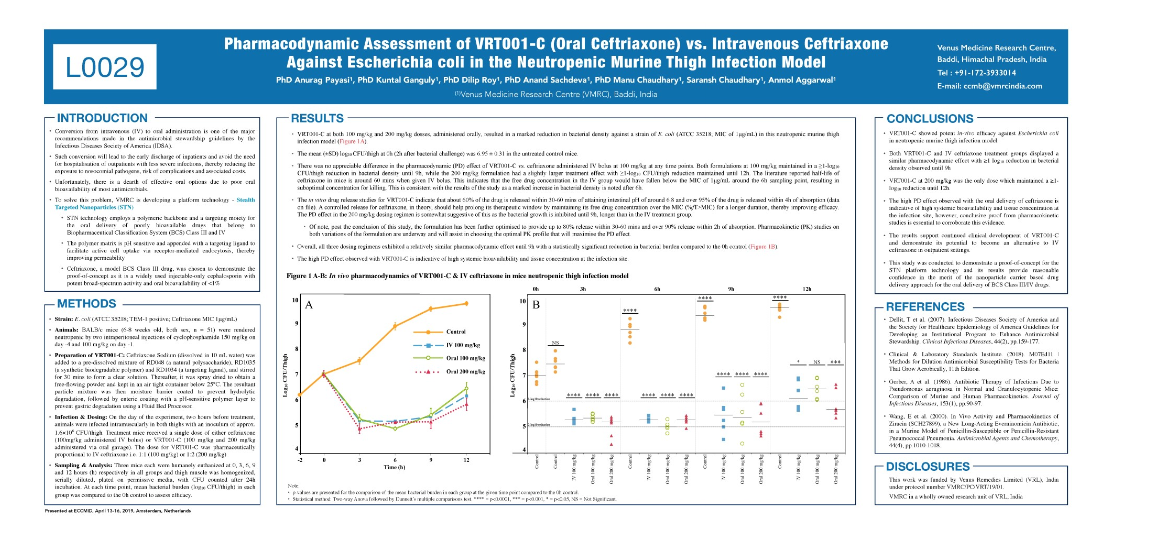
According to the results of this study, VRT001-C showed potent in-vivo efficacy against Escherichia coli in a neutropenic murine thigh infection model. Further, both VRT001-C and IV ceftriaxone treatment groups displayed a similar pharmacodynamic effect with ≥1 log 10 reductions in bacterial density observed until 9h. Furthermore, VRT001-C at 200 mg/kg was the only dose which maintained a ≥1-log 10 reduction until 12h. As per the results of this study high PD effect observed with the oral delivery of ceftriaxone is indicative of high systemic bioavailability and tissue concentration at the infection site, however, conclusive proof from pharmacokinetic studies is essential to corroborate this evidence.
The results support continued clinical development of VRT001-C and demonstrate its potential to become an alternative to IV ceftriaxone in outpatient settings. This study was conducted to demonstrate a proof-of-concept for the STN platform technology and its results provide reasonable confidence in the merit of the nanoparticle carrier based drug delivery approach for the oral delivery of BCS Class III/IV drugs.